Digital PLM Replaces Document-Based Processes
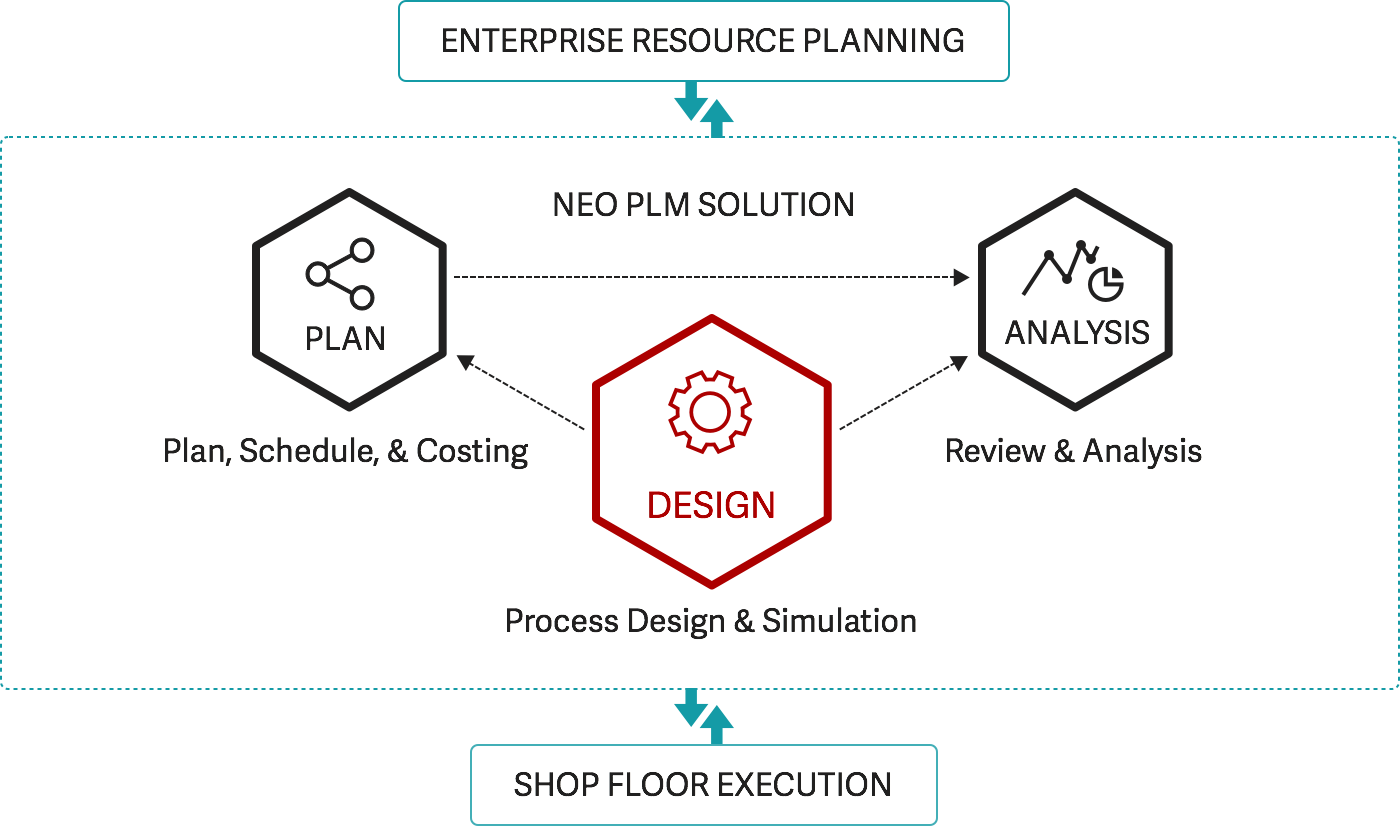
Neo’s Process PLM solution represents a technological breakthrough for pharmaceutical companies and other process manufacturers. For the first time, process design technology integrates seamlessly with manufacturing execution systems. The result: a unique and powerful digital-design-based approach to managing information, productivity and quality throughout the product lifecycle.
Implement Quality by Design (QbD) and Process Validation
Neo’s PLM technology ensures all quality attributes are captured and tracked throughout the product lifecycle, enabling faster technology transfer and process validation. Analysis tools provide instant access to process deviations, allowing quality issues to be resolved more quickly.
Anticipate the impact of process changes
Neo created the only PLM solution that allows you to model the full impact of process changes across the lifecycle before manufacturing begins.
Facilitate scale-up and production planning
In late-stage research, Neo’s Process PLM solution allows you to create a full engineering design of the process using a powerful and intuitive user interface. Comprehensive simulation performs detailed calculations, providing engineers with rich decision information including:
- Manufacturing time-cycle
- Critical path analysis
- Material and energy balances
- Compliance verification

Optimize processes and product quality
After a batch is produced, execution data automatically maps back to the process design—enabling easy point-and-click data analysis.
- Continuously improve quality assurance and cost optimization
- Easily detect discrepancies
- Move toward true exception-based quality management
Streamline and automate manufacturing processes
Neo’s Process PLM software integrates with—and complements—your existing systems. It is the only PLM solution in the marketplace that enables process design to automatically create shop-floor manufacturing documents, as well as configure and integrate recipes directly into execution systems.
- Synchronize processes and data across systems
- Improve technology transfer between facilities
- Reduce costs and eliminate errors

Capture and reuse formulations and recipes
This includes flexible and dynamic change control to manage revisions and the ability to configure and track formulation and recipe variants. Process designs created in one plant can be easily transferred to other plants.
